

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM50148732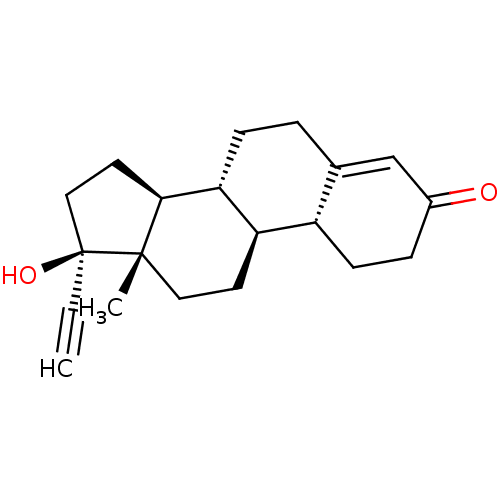 (17-ethynyl-17beta-hydroxyestr-4-en-3-one | 17alpha...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Inc. Curated by ChEMBL | Assay Description Dissociation constant for progesterone receptor | J Med Chem 47: 3381-7 (2004) Article DOI: 10.1021/jm030640n BindingDB Entry DOI: 10.7270/Q2KP81NR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (RAT-Rattus norvegicus) | BDBM50148732 (17-ethynyl-17beta-hydroxyestr-4-en-3-one | 17alpha...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Equilibrium dissociation constant for rat uterine estrogen receptor binding [3H]estradiol | J Med Chem 27: 1131-7 (1984) BindingDB Entry DOI: 10.7270/Q2W95CDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sex hormone-binding globulin (Homo sapiens (Human)) | BDBM50148732 (17-ethynyl-17beta-hydroxyestr-4-en-3-one | 17alpha...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
University of British Columbia Curated by ChEMBL | Assay Description Displacement of [3H]5alpha dihydrotestosterone from human sex hormone binding globulin | J Med Chem 51: 2047-56 (2008) Article DOI: 10.1021/jm7011485 BindingDB Entry DOI: 10.7270/Q2RX9DC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||